Reimagining spinal fusion
Be at the forefront of lumbar spinal fusion innovation

Redefining joint stabilization for surgeons and patients
Zygofix is committed to providing safe and effective fixation solutions specifically designed for the rapidly growing Ambulatory Surgery Center (ASC) market.
Our innovative technology, developed by renowned orthopedic experts responsible for several groundbreaking advancements, sets a new benchmark in joint stabilization.

zLOCK technology
Small spinal implant
creating a natural bridge
zLOCK, is the only implant that conforms to the facet anatomy during insertion, providing comparable stability to a pedicle screw construct through a miniature bendable internal facet fixation device.
* FDA cleared system consists of the zLOCK spacer and zLOCK trans-facet screw
Which one would you prefer in your spine?
* FDA cleared system consists of the zLOCK spacer and zLOCK trans-facet screw
The zLOCK system is based on a miniature implant, providing an alternative fixation solution to pedicle screws. zLOCK enables joint stabilization with minimal surgical trauma, reducing the risk of long-term complications such as adjacent segment degeneration.
Innovative fixation solution

3D Printed bendable titanium implant
The zLOCK system is based on a miniature 3D Printed bendable titanium implant designed to stabilize the spine by anchoring within the facet joint.
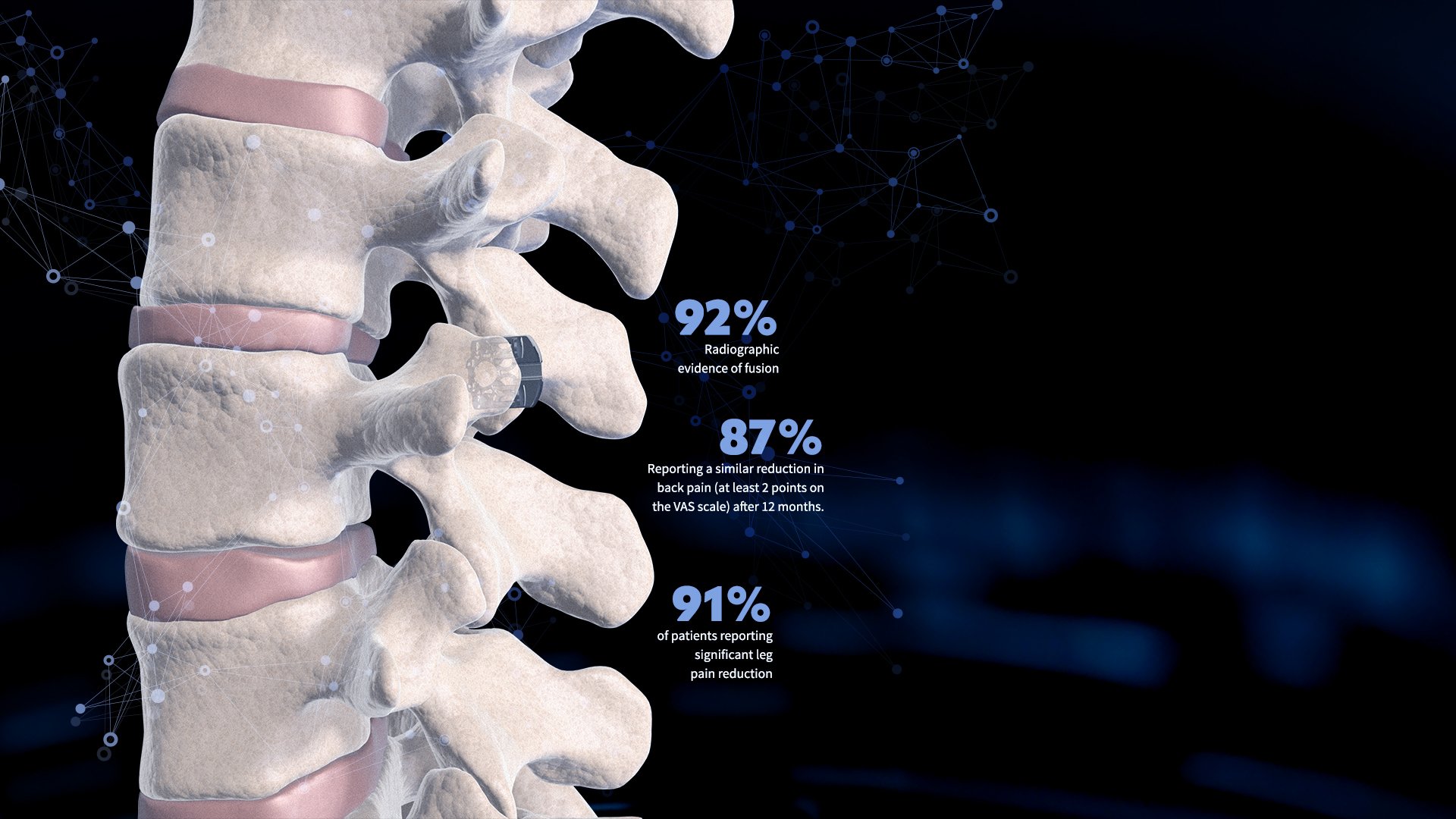
Clinically validated
The zLOCK system has been clinically tested in the EU under clinical studies since 2018, showing long-term pain reduction and radiographic signs of bone growth.
Its design minimizes impact and trauma to adjacent segments, reducing the risk of complications.
* FDA cleared system consists of the zLOCK spacer and zLOCK trans-facet screw
Why choose zLOCK?
The natural choice for ASC spinal fusion

Management
-
Medical devices executive with over 20 years of experience in engineering, product and regulatory domains
-
Serial entrepreneur in the field of spinal implants with over 30 years’ experience; 29 issued and 43 pending patents.
-
Medical Director, Chairman & Director, Israel Spine Center, Assuta Hospital, President, Israel Orthopedic Association;
Scientific Advisory Board
-
PharmD and MD from the University of Maryland; Postdoc Orthopedic surgery training at St. Joseph Medical Center.
-
Expert in minimally invasive orthopedic techniques. UCLA Neuroscience; Orthopedic Surgery at Stanford.
Award winning technology